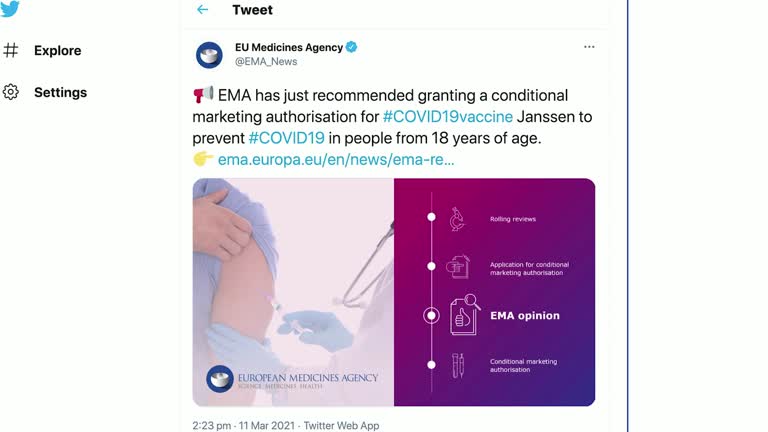
The European Union’s drugs regulator on Thursday (March 11) recommended conditionally approving Johnson & Johnson’s single dose COVID-19 vaccine, as the bloc seeks to speed up a stuttering inoculation campaign and boost its supplies of vaccines. .
European Health Commissioner Stella Kyriakides said the formal authorisation of Johnson & Johnson’s COVID-19 vaccine would follow shortly.
“This marks another key step towards ensuring that all citizens can access safe and effective vaccinations as soon as possible,” Kyriakides wrote on Twitter.
The COVID-19 shot is the fourth to be endorsed for use in the EU after vaccines from Pfizer-BioNTech, AstraZeneca-Oxford University and Moderna, and is recommended for those over 18 years of age, the European Medicines agency (EMA) said.
The United States, Canada and Bahrain have also approved the shot. South Africa is carrying out an expedited review.